

 Leaching
of nitrate on sandy soils in southwestern Indiana has likely been substantial
this spring unless early season fertilizer applications utilized a nitrification
inhibitor.
Leaching
of nitrate on sandy soils in southwestern Indiana has likely been substantial
this spring unless early season fertilizer applications utilized a nitrification
inhibitor. Nitrogen (N) is often the most limiting nutrient for corn production and many factors must be considered when determining the right amount of N to apply for optimum economic yields. ne factor is how much N has been lost before the crop can accumulate it. Spring rainfall in particular can reduce the amount of N remaining in the rootzone from pre-plant and at-planting fertilizer applications. The recent rains have many folks wondering how much N has been lost from these applications. While we can not give you field-specific estimates of N loss, we can offer some guidelines to help you estimate these losses based on regional soil temperatures and rainfall
The form of N present in the soil determines its susceptibility to loss. Nitrogen in the ammonium form is attracted to the soil cation exchange sites so it does not leach below the rootzone. Nor is ammonium subject to a process called denitrification [when nitrate nitrogen is changed into various gas forms and lost to the atmosphere]. If N stayed in the ammonium form then N losses would be minimal no matter how much it rained. Unfortunately that is not the case. Ammonium is normally converted to nitrate by soil bacteria and nitrate is both leachable in sandy soils and subject to denitrification in poorly drained soils. The amount of N lost from soils depends on when the ammonium changes to nitrate, how much rainfall occurs after the nitrate is formed, the water holding characteristics of the soil, the soil temperature when the soil is saturated and the length of time the soil remains saturated.
Although we know the amount of N we already have applied, when it was applied, and what form it was in, we only can make educated guesses on how fast the ammonium changed to nitrate and how much nitrate has been lost.
 Nitrogen
applied as anhydrous ammonia is all ammonium once it dissolves in the soil water.
Anhydrous is toxic to the ammonium-converting bacteria in the injection zone
and it takes about 2 weeks for them to repopulate the application zone soil
and begin converting ammonium to nitrate. Urea ammonium nitrate (UAN) solutions
are more susceptible to leaching and denitrification than anhydrous because
25% of the N at application is already in the nitrate form. Also, the conversion
of ammonium to nitrate begins immediately after application if temperatures
are warm because the UAN does not reduce soil bacteria populations. Conversion
of ammonium to nitrate from urea is the same as that from UAN.
Nitrogen
applied as anhydrous ammonia is all ammonium once it dissolves in the soil water.
Anhydrous is toxic to the ammonium-converting bacteria in the injection zone
and it takes about 2 weeks for them to repopulate the application zone soil
and begin converting ammonium to nitrate. Urea ammonium nitrate (UAN) solutions
are more susceptible to leaching and denitrification than anhydrous because
25% of the N at application is already in the nitrate form. Also, the conversion
of ammonium to nitrate begins immediately after application if temperatures
are warm because the UAN does not reduce soil bacteria populations. Conversion
of ammonium to nitrate from urea is the same as that from UAN.
Conversion rates of ammonium to nitrate are highly dependent on soil temperature.
Little conversion occurs when soils are below 50 °F. At temperatures near
60 °F complete conversion is estimated to take less than 14 days and at
greater than 70 °F, less than 7 days. Soil temperatures in southern and
central Indiana have been above 60 °F on a regular basis since April 15th
(Figure 1a). Average soil temperatures at the 4" depth this spring were
greater than 60 °F on 29, 21, 21, and 18 days at the Purdue Agricultural
Centers (PACs) in Vincennes, Butlerville, Lafayette, and Farmland, respectively.
Thus, significant amount of N have been converted to nitrate in these areas
already. 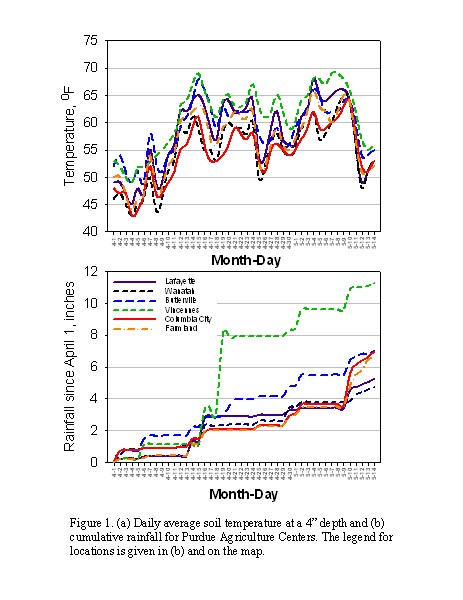 In
northern Indiana at the PACs in Wanatah and Columbia City there have been only
8 and 6 days with average soil temperatures above 60 °F so much of the N
applied as ammonium may still be in the ammonium form.
In
northern Indiana at the PACs in Wanatah and Columbia City there have been only
8 and 6 days with average soil temperatures above 60 °F so much of the N
applied as ammonium may still be in the ammonium form.
Nitrification inhibitors, such as nitrapyrin, dicyandiamide, and ammonium thiosulfate, slow the conversion of ammonium to nitrate by affecting the soil bacteria. Conversion of ammonium to nitrate is limited for approximately 4-6 weeks after application with nitrapyrin or dicyandiamide. Ammonium thiosulfate may also slow conversion about 2-3 weeks.
At the time of these last rains at the end of the 2nd week of May, most of the ammonium applied in northern Indiana was probably still in the ammonium form and not subject to leaching or denitrification. Because of warmer soil temperatures in central and southern Indiana, a significant amount of the N applied as ammonium without a nitrification inhibitor had likely been converted to nitrate and has a high potential for leaching and denitrification losses. Ammonium-N applied in April or later with an inhibitor is likely still in the ammonium form.
Leaching from sandy soils
Once nitrate is present in the soil its leaching is not temperature dependent. How deep in the soil profile the nitrate moves depends on the water holding capacity of the soil, how wet the soil is when it begins raining, and the amount of rainfall. A typical sandy soil in Indiana holds about 1-2 inches of rain in each foot of soil. Therefore the movement of nitrate downward is approximately 6 to 12 inches per inch of rainfall if the soil is at field capacity when it begins raining.
Rainfall since the beginning of April has likely caused significant leaching in sandy soils in northwest and southwest Indiana where sandy soils are prevalent (Figure 1b). In southwest Indiana, the Vincennes area has had nearly 12 inches of rainfall. Nitrate in sandy soils in this region was probably leached beyond the maximum rootzone of corn – 3 feet or more. The 4.7 inches of rain at Wanatah in northwest Indiana is likely to have caused substantial nitrate leaching from the rootzone as well.
Denitrification in poorly-drained soils
Spring rainfall has been sufficient to saturate poorly-drained soils in most of Indiana. Denitrification will take place in saturated soils if nitrate is present and soil temperatures are warm enough. Denitrification is negligible below 50 °F but increases to as much as 5% of the nitrate-N per day of saturation at soil temperatures of 70 °F.
Temperatures in southern and central Indiana have been high enough to result in some denitrification in poorly-drained soils, but substantial nitrate losses are unlikely because soil temperatures at most locations were around 55 °F or lower when the rainy period began on May 1 and 11.
One way to get a somewhat better estimate of soil N supply is to take a soil sample and have it analyzed for nitrate. The later in the season this is done the better. Standard recommendations suggest sampling when corn is at the 4- to 6-leaf stage; hence it is called the pre-sidedress soil nitrate test or PSNT. The best situation to utilize this procedure is when manure has been broadcast in the fall or early spring. Specific recommendations for preplant N have not been developed and use of the procedure with banded manure or fertilizers is difficult because of sampling issues. Because of the variability in soil N across fields a sampling area of no more than 10 uniform acres is recommended and each sample should consist of 15-25 cores taken 1 foot deep. You should dry the sample before mailing to a soil testing laboratory so your results will reflect the soil condition when it was sampled.
Results of the soil nitrate test are typically reported in parts per million
(ppm) or milligrams per kilogram (mg/kg) which are equivalent in value. If more
than 25 ppm nitrate-N is found in the sample then no additional N is recommended.
This amount of nitrate is unlikely unless a high rate of preplant fertilizer
or manure has been applied. At lower levels of nitrate-N, adjustments can be
made to sidedress N rates. If little nitrate is found it might indicate that
ammonium had not yet been converted to nitrate as well as indicating loss of
nitrate from the rootzone so some interpretation of the results is needed. More
information on the PSNT can be obtained at:
http://www.agry.purdue.edu/ext/pubs/AY-314-W.pdf.
Leaching of nitrate on sandy soils in southwestern Indiana has likely been substantial this spring unless early season fertilizer applications utilized a nitrification inhibitor. Some of the nitrate applied to sandy soils in northern Indiana has probably been lost from the rootzone as well. However, fertilizer added as ammonium in cold northern Indiana soils was likely still in the ammonium form when the rains came and most of the applied ammonium likely still remains in the rootzone.
Loss of nitrate via denitrification in poorly-drained soils has probably been
minimal in northern Indiana and low in central to southern Indiana. Although
warm soil temperatures promoted conversion of ammonium to nitrate in late April
and early May in central and southern Indiana, these soils were relatively cold
(<55 °F) during the rainy periods and this likely limited the amount
of denitrification that occurred.
© 2010 , Purdue University, an equal access, equal opportunity university. This material may be available in alternative formats. If you have trouble accessing this page because of a disability, please contact RLNielsen at rnielsen@purdue.edu.